Abstract
Background:As chimeric antigen receptor-T cell (CAR-T) therapy continue to be developed to treat hematologic malignancies, it is important to assess whether the trials in which CAR-T are investigated include a representative sample of the impacted populations. The purpose of this study is to describe the geographic and demographic characteristics of pivotal clinical trials for CAR-T cell therapy and the prevalence of the related hematological malignancies in the United States.
Methods: Adult CAR-T CTs were collected from the US Food and Drug Administration (FDA) Databases 'Oncology (cancer)/Hematologic Malignancies approval between January 2017 to May 2022. Trials were also reviewed for demographic data on ClinicalTrials.gov (CT.gov) and Primary Literature related to drug approval. Prevalence data from 2018 was extracted from Surveillance, Epidemiology, and End Results (SEER) Program by race, sex, and ethnicity for Non-Hodgkin's Lymphoma (NHL) (including diffuse large B cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and follicular lymphoma (FL)), acute lymphoblastic leukemia (ALL), and multiple myeloma (MM). We conducted bivariate analyses using Pearson's chi-squared tests for the categorical racial variables.
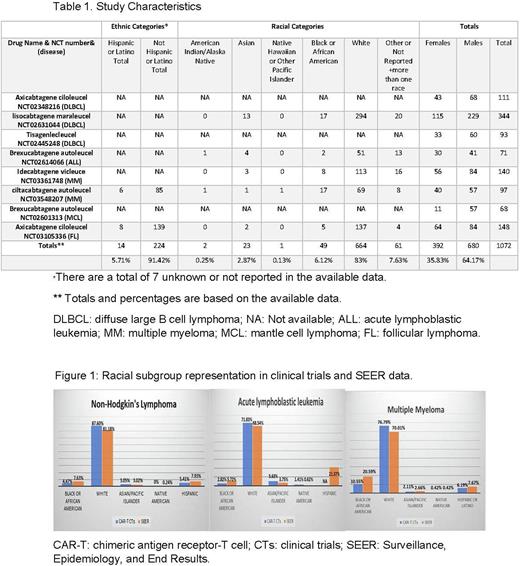
Results:A total of 9 products were approved: 3 for DLBCL (tisagenlecleucel, axicabtagene ciloleucel, and lisocabtagene maraleucel), 2 for ALL (brexucabtagene autoleucel and tisagenlecleucel, we excluded tisagenlecleucel which was approved for patients up to age 25), 2 for MM (idecabtagene vicleucel, and ciltacabtagene autoleucel), and 1 product each was approved for MCL (brexucabtagene autoleucel), and FL (axicabtagene ciloleucel). We found 8 studies with manuscripts that matched the clinical trials that led to the FDA approval (Table 1). All the products are approved for relapsed or refractory disease. The median number of states included in the 8 studies was 12 (range, 9-27), the median number of locations was 16 (range 14-63), and 7/8 (88%) studies had international locations. All studies were performed in a total of 30 states. Excluding idecabtagene vicleuce for MM, which had 27 states, and 63 sites (21 states with more than one location), the maximum number of states with more than one location was 6 in one study.
While 5/8 ( 63%) studies reported racial categories, only 2 (25%) reported ethnic categories (ciltacabtagene autoleucel for MM, and axicabtagene ciloleucel for FL). DLBCL studies had 548 patients, with one of 3 studies (lisocabtagene maraleucel) reporting racial categories in 344 patients. These included 13 (4%) Asian/Pacific Islander, 17 (5%) Black/African American, 294 (85%) Whites, and 20 not reported. ALL study had 71 patients, with 4 (6%) Asian/Pacific Islander, 2 (3%) Black/African American, 1 (1%) American Indian, 51 (72%) Whites, and 13 (18%) did not report. MM studies had 237, with 5 (2%) Asian/Pacific Islander, 25 (11%) Black/African American, 1 (0.5%) American Indian, 182 (77%) Whites, 10 more than one race, and 14 (6%) did not report. MCL study evaluated 68 (57 male and 11 women) without reporting racial or ethnic categories. The CAR T cell for follicular study had 148 patients, with 2 (1%) Asian/Pacific Islander, 5 (3%) Black/African American, 137 (93%) Whites, 4 (3%) did not report, there were only 8 (5%) Hispanic or Latino patients. All studies described the overall sex distribution, with 392 (35.8%) women and 680 (64.2%) men across the 8 studies. Sex was not differentiated by race in any of the studies. Figure 1 shows lower representation for Black/African Americans. Chi-squared scores were 20,1, and 14.8 for African Americans representation's compared to other races in NHL, ALL, and MM respectively (P values' 0.0001, 0.3, and 0.0001, respectively). Asian/Pacific Islanders representation did not differ from the SEER population in all diseases. There was under-representation for Hispanics in ethnic categories in the 2 studies (5.7%) which reported ethnic percentages.
Conclusion:There is a low enrollment of Black/African American patients in trials for CAR-T therapy, and that the disparity is substantial, especially for NHL and MM. The misrepresentation of minorities in CAR-T CTs may lead to results that may not fully translate to such populations. These disparities in enrollment should be corrected in future studies. CAR-T CTs manuscripts and CT.gov should prioritize having all-inclusive demographic reporting and representation.
Disclosures
Cortes:Gilead: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Sun Pharma: Consultancy, Research Funding; Biopath Holdings Inc: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapeutic: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal